Sharp debridement is a foundational part of wound bed preparation, but it may need to be minimized due to pain and or clinical considerations. In some cases, sharp debridement may also be contraindicated altogether, including situations such as unstageable wounds or suspected deep tissue injury (DTI), or patients with bleeding disorders such as hemophilia. In those situations, clinicians still need a reliable way to support wound healing and maintain progress without increasing procedural burden.
When sharp debridement is limited, clinicians may rely on alternatives such as autolytic debridement with moisture-retentive dressings or enzymatic debridement. While these approaches can be effective, many teams still need a solution that is consistent, well-tolerated, and clinically proven without adding discomfort to the patient.
This is where non-contact wound therapy, such as Sanuwave UltraMIST, becomes especially valuable.
WHAT IS NON-CONTACT WOUND THERAPY?
Non-contact wound therapy is a type of wound treatment that supports healing without directly touching the wound bed with a device or instrument. Instead of contact-based tools, it uses a method that can help manage the wound environment while minimizing pain, irritation, and disruption to fragile tissue.
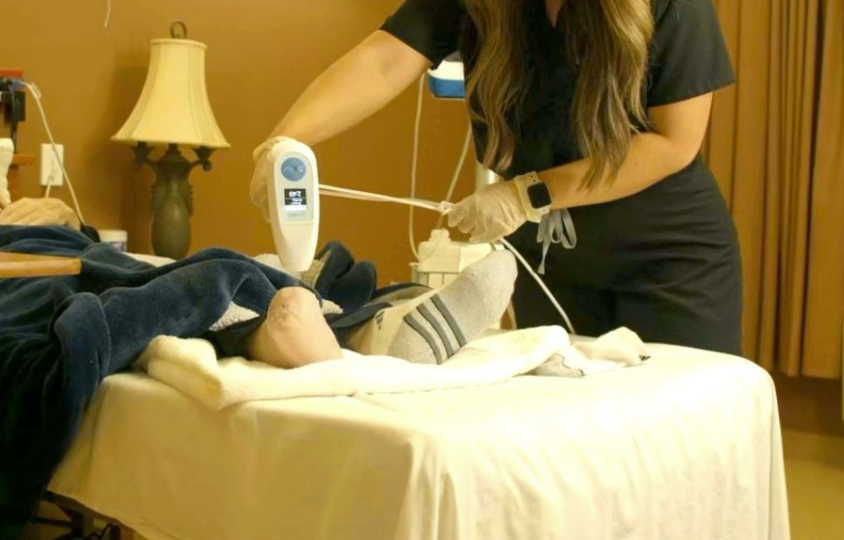
HOW DOES ULTRAMIST THERAPY WORK AS A NON-CONTACT WOUND THERAPY?
Sanuwave UltraMIST Therapy is a clinically proven treatment that delivers low energy ultrasound to your wound. A fluid/saline mist is used to deliver the ultrasound, so there is no direct contact to the wound.
The UltraMIST System’s acoustic wave therapy promotes healing by controlling inflammation and reducing bacteria in the wound bed while increasing angiogenesis. Further promoting healing, it increases perfusion through vasodilation, ultimately increasing oxygen and nutrients to the tissue.
Because it does not require contact with the wound, Sanuwave UltraMIST is painless to apply. Some studies have even shown reduced pain after UltraMIST Therapy is applied. ¹,²

WHAT ARE THE KEY CLINICAL CONSIDERATIONS FOR Sanuwave ULTRAMIST?
UltraMIST Therapy is used to support key aspects of wound progression across many chronic and acute wounds, including cases with:
- Wound bed preparation needs
- Excessive bioburden
- Poor granulation tissue
- Delayed epithelialization
- Patients seeking non-invasive options.
HOW IS sanuwave ULTRAMIST THERAPY PERFORMED IN A WOUND CARE CLINIC?
Sanuwave UltraMIST Therapy is designed to seamlessly integrate into clinical workflows, helping wound care teams follow a consistent protocol from visit to visit. Using UltraMIST 2–3 times per week is considered reasonable and medically necessary per Medicare guidelines. For more detailed treatment guidance, the OX BioMed team provides onboarding support and device training to help your staff implement UltraMIST Therapy with confidence.
CAN sanuwave ULTRAMIST BE USED ALONGSIDE STANDARD WOUND CARE TREATMENTS?
Yes. Sanuwave UltraMIST Therapy is commonly integrated into existing wound care protocols based on clinician judgment, wound type, and facility standards.
ULTRAMIST THERAPY ORDERING AND IMPLEMENTATION SUPPORT
Are you dealing with wounds that are too painful for sharp debridement? Our team provides the resources clinics need to implement UltraMIST Therapy with confidence and maintain consistent use over time, including:
- Ordering Sanuwave UltraMIST devices and disposables
- Onboarding, device training, and ongoing support
- Dedicated market access support, including fast in-house insurance verification, prior authorizations, and documentation education
To get started, contact us here.
Sources:
- Gibbons GW, Orgill DP, Serena T, et al. A prospective, randomized, controlled trial comparing the effects of noncontact, low-frequency ultrasound to standard care in healing venous leg ulcers. Ostomy Wound Manage. 2015;61(6):16-29.
- Driver VR, Yao M, Miller CJ. Noncontact low-frequency ultrasound therapy in the treatment of chronic wounds: a meta-analysis. Wound Repair Regen. 2011;19(4):475-480. doi:10.1111/j.1524-475X.2011.00701.x
- SANUWAVE Health, Inc. UltraMIST® Therapy. Accessed January 26, 2026. https://sanuwave.com/
- Approved SANUWAVE UltraMIST® marketing materials. OX BioMed authorized retailer content.
